COVID-19 Statistics
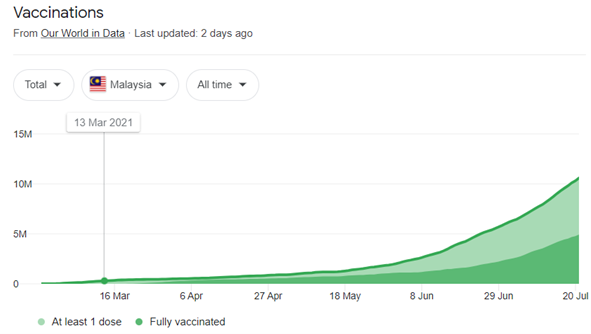
Equitable access to safe and effective vaccines is vital to eliminating the COVID-19 pandemic, so seeing so many vaccines in testing and development is quite encouraging. Safe and efficient vaccines are a game-changing weapon, but for the time being, we must continue to wear masks, wash our hands, ensure proper ventilation indoors, and physically distance ourselves from and avoid crowds. Being vaccinated does not allow us to disregard caution and endanger ourselves and others, especially when research into how well vaccines protect not only against sickness but also against infection and transmission is underway. But it is vaccination, not vaccines, that will put an end to the epidemic. We must assure fair and equitable access to vaccines, as well as that every country obtains them and can use them to safeguard its citizens, beginning with the most vulnerable.